Periodic Table Element Comparison: Compare Elements - Neon vs Oxygen
Compare Neon and Oxygen on the basis of their properties, attributes and periodic table facts. Compare elements on more than 90 properties. All the elements of similar categories show a lot of similarities and differences in their chemical, atomic, physical properties and uses. These similarities and dissimilarities should be known while we study periodic table elements. You can study the detailed comparison between Neon vs Oxygen with most reliable information about their properties, attributes, facts, uses etc. You can compare Ne vs O on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure, Electronaffinity, physical states, electrical conductivity and many more. Neon and Oxygen comparison table on more than 90 properties.
Neon and Oxygen Comparison
Facts
| Name | Neon | Oxygen |
|---|---|---|
| Atomic Number | 10 | 8 |
| Atomic Symbol | Ne | O |
| Atomic Weight | 20.1797 | 15.9994 |
| Phase at STP | Gas | Gas |
| Color | Colorless | Colorless |
| Metallic Classification | Noble Gas | Other Nonmetal |
| Group in Periodic Table | group 18 | group 16 |
| Group Name | helium family or neon family | oxygen family |
| Period in Periodic Table | period 2 | period 2 |
| Block in Periodic Table | p -block | p -block |
| Electronic Configuration | [He] 2s2 2p6 | [He] 2s2 2p4 |
| Electronic Shell Structure (Electrons per shell) | 2, 8 | 2, 6 |
| Melting Point | 24.56 K | 54.8 K |
| Boiling Point | 27.07 K | 90.2 K |
| CAS Number | CAS7440-01-9 | CAS7782-44-7 |
| Neighborhood Elements | Neighborhood Elements of Neon | Neighborhood Elements of Oxygen |
History
| Parameter | Neon | Oxygen |
|---|---|---|
| History | The element Neon was discovered by W. Ramsay and W. Travers in year 1898 in United Kingdom. Neon derived its name from the Greek neos, meaning 'new'. | The element Oxygen was discovered by W. Scheele in year 1771 in Sweden and United Kingdom. Oxygen derived its name from the Greek word oxy-, both 'sharp' and 'acid', and -gen, meaning 'acid-forming'. |
| Discovery | W. Ramsay and W. Travers (1898) | W. Scheele (1771) |
| Isolated | W. Ramsay and W. Travers (1898) | W. Scheele (1771) |
Presence: Abundance in Nature and Around Us
Parts per billion (ppb) by weight / by atoms (1ppb =10^-7 %)
| Property | Neon | Oxygen |
|---|---|---|
| Abundance in Universe | 1300000 / 80000 | 10000000 / 800000 |
| Abundance in Sun | 1000000 / 70000 | 9000000 / 700000 |
| Abundance in Meteorites | - / - | 410000000 / 480000000 |
| Abundance in Earth's Crust | 3.0 / 3 | 460000000 / 600000000 |
| Abundance in Oceans | 0.12 / 0.037 | 857000000 / 331000000 |
| Abundance in Humans | - / - | 610000000 / 240000000 |
Crystal Structure and Atomic Structure
| Property | Neon | Oxygen |
|---|---|---|
| Atomic Volume | 22.42 cm3/mol | 22.4134 cm3/mol |
| Atomic Radius | 38 pm | 48 pm |
| Covalent Radius | 69 pm | 73 pm |
| Van der Waals Radius | 154 pm | 152 pm |
Atomic Spectrum - Spectral Lines | ||
| Emission Spectrum |  |  |
| Absorption Spectrum |  |  |
| Lattice Constant | 442.9, 442.9, 442.9 pm | 540.3, 342.9, 508.6 pm |
| Lattice Angle | π/2, π/2, π/2 | π/2, 2.313085, π/2 |
| Space Group Name | Fm_ 3m | C12/m1 |
| Space Group Number | 225 | 12 |
| Crystal Structure | Face Centered Cubic 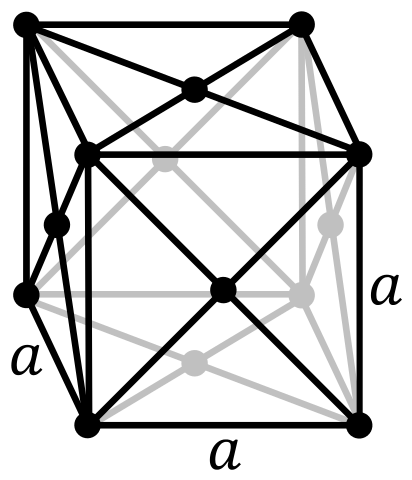 | Base Centered Monoclinic 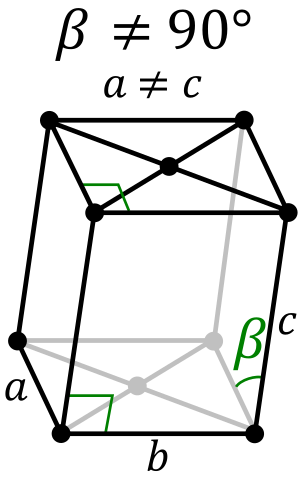 |
Atomic and Orbital Properties
| Property | Neon | Oxygen |
|---|---|---|
| Atomic Number | 10 | 8 |
| Number of Electrons (with no charge) | 10 | 8 |
| Number of Protons | 10 | 8 |
| Mass Number | 20.1797 | 15.9994 |
| Number of Neutrons | 10 | 8 |
| Shell structure (Electrons per energy level) | 2, 8 | 2, 6 |
| Electron Configuration | [He] 2s2 2p6 | [He] 2s2 2p4 |
| Valence Electrons | 2s2 2p6 | 2s2 2p4 |
| Oxidation State | - | -2 |
| Atomic Term Symbol (Quantum Numbers) | 1S0 | 3P2 |
| Shell structure | 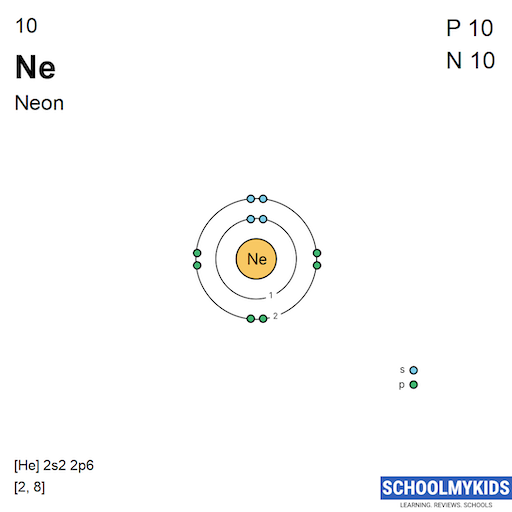 | 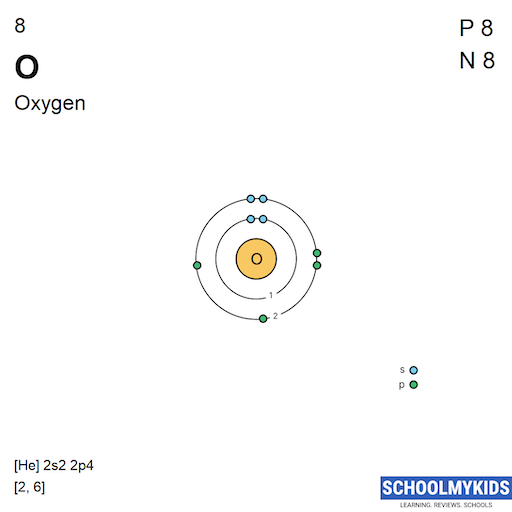 |
Isotopes and Nuclear Properties
Neon has 3 stable naturally occuring isotopes while Oxygen has 3 stable naturally occuring isotopes.
| Parameter | Neon | Oxygen |
|---|---|---|
| Known Isotopes | 16Ne, 17Ne, 18Ne, 19Ne, 20Ne, 21Ne, 22Ne, 23Ne, 24Ne, 25Ne, 26Ne, 27Ne, 28Ne, 29Ne, 30Ne, 31Ne, 32Ne, 33Ne, 34Ne | 12O, 13O, 14O, 15O, 16O, 17O, 18O, 19O, 20O, 21O, 22O, 23O, 24O, 25O, 26O, 27O, 28O |
| Stable Isotopes | Naturally occurring stable isotopes: 20Ne, 21Ne, 22Ne | Naturally occurring stable isotopes: 16O, 17O, 18O |
| Neutron Cross Section | 0.04 | 0.00028 |
| Neutron Mass Absorption | 0.0006 | 0.000001 |
Chemical Properties: Ionization Energies and electron affinity
| Property | Neon | Oxygen |
|---|---|---|
| Valence or Valency | 0 | 2 |
| Electronegativity | - | 3.44 Pauling Scale |
| Electron Affinity | 0 kJ/mol | 141 kJ/mol |
| Ionization Energies | 1st: 2080.7 kJ/mol 2nd: 3952.3 kJ/mol 3rd: 6122 kJ/mol 4th: 9371 kJ/mol 5th: 12177 kJ/mol 6th: 15238 kJ/mol 7th: 19999 kJ/mol 8th: 23069.5 kJ/mol 9th: 115379.5 kJ/mol 10th: 131432 kJ/mol | 1st: 1313.9 kJ/mol 2nd: 3388.3 kJ/mol 3rd: 5300.5 kJ/mol 4th: 7469.2 kJ/mol 5th: 10989.5 kJ/mol 6th: 13326.5 kJ/mol 7th: 71330 kJ/mol 8th: 84078 kJ/mol |
Physical Properties
| Property | Neon | Oxygen |
|---|---|---|
| Density | 0.0009 g/cm3 | 0.001429 g/cm3 |
| Molar Volume | 22.42 cm3/mol | 22.4134 cm3/mol |
Elastic Properties | ||
| Young Modulus | - | - |
| Shear Modulus | - | - |
| Bulk Modulus | - | - |
| Poisson Ratio | - | - |
Hardness - Tests to Measure of Hardness of Element | ||
| Mohs Hardness | - | - |
| Vickers Hardness | - | - |
| Brinell Hardness | - | - |
Electrical Properties | ||
| Electrical Conductivity | - | - |
| Resistivity | - | - |
| Superconducting Point | - | - |
Heat and Conduction Properties | ||
| Thermal Conductivity | 0.0491 W/(m K) | 0.02658 W/(m K) |
| Thermal Expansion | - | - |
Magnetic Properties | ||
| Magnetic Type | Diamagnetic | Paramagnetic |
| Curie Point | - | - |
| Mass Magnetic Susceptibility | -4.1e-9 m3/kg | 0.000001335 m3/kg |
| Molar Magnetic Susceptibility | -8.27e-11 m3/mol | 4.27184e-8 m3/mol |
| Volume Magnetic Susceptibility | -3.69e-9 | 0.00000190772 |
Optical Properties | ||
| Refractive Index | 1.000067 | 1.000271 |
Acoustic Properties | ||
| Speed of Sound | 936 m/s | 317.5 m/s |
Thermal Properties - Enthalpies and thermodynamics
| Property | Neon | Oxygen |
|---|---|---|
| Melting Point | 24.56 K | 54.8 K |
| Boiling Point | 27.07 K | 90.2 K |
| Critical Temperature | 44.4 K | 154.59 K |
| Superconducting Point | - | - |
Enthalpies | ||
| Heat of Fusion | 0.34 kJ/mol | 0.222 kJ/mol |
| Heat of Vaporization | 1.75 kJ/mol | 3.41 kJ/mol |
| Heat of Combustion | - | - |
Regulatory and Health - Health and Safety Parameters and Guidelines
| Parameter | Neon | Oxygen |
|---|---|---|
| CAS Number | CAS7440-01-9 | CAS7782-44-7 |
| RTECS Number | RTECSQP4450000 | RTECSRS2060000 |
| DOT Hazard Class | 2.2 | 2.2 |
| DOT Numbers | 1913 | 1073 |
| EU Number | - | - |
| NFPA Fire Rating | - | 0 |
| NFPA Health Rating | - | 3 |
| NFPA Reactivity Rating | - | 2 |
| NFPA Hazards | - | Oxidizing Agent |
| AutoIgnition Point | - | - |
| Flashpoint | - | - |
Compare With Other Elements
Compare Neon and Oxygen with other elements of the periodic table.
Compare Neon with all Group 18 elementsCompare Neon with HeliumCompare Neon with ArgonCompare Neon with KryptonCompare Neon with XenonCompare Neon with RadonCompare Neon with Oganesson Compare Neon with all Period 2 elementsCompare Neon with LithiumCompare Neon with BerylliumCompare Neon with BoronCompare Neon with CarbonCompare Neon with NitrogenCompare Neon with OxygenCompare Neon with Fluorine Compare Neon with all Noble Gas elements | Compare Oxygen with all Group 16 elementsOxygen vs Sulfur ComparisonOxygen vs Selenium ComparisonOxygen vs Tellurium ComparisonOxygen vs Polonium ComparisonOxygen vs Livermorium Comparison Compare Oxygen with all Period 2 elementsOxygen vs Lithium ComparisonOxygen vs Beryllium ComparisonOxygen vs Boron ComparisonOxygen vs Carbon ComparisonOxygen vs Nitrogen ComparisonOxygen vs Fluorine ComparisonOxygen vs Neon Comparison Compare Oxygen with all Other Nonmetal elements |

