This chapter explores the fascinating phenomenon of how electricity can induce chemical changes in substances.
I. Introduction
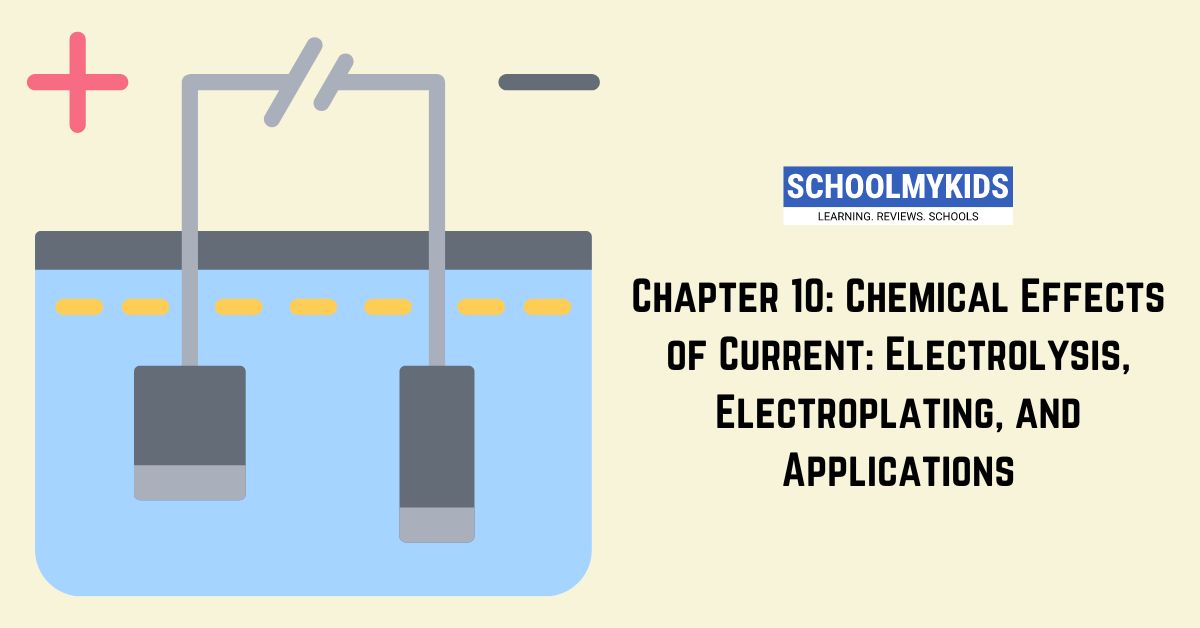
- When an electric current is passed through a specific type of liquid or molten substance called an electrolyte, it can cause a chemical reaction to occur. This process is called electrolysis.
- Electrolytes contain dissolved ionic compounds that allow the flow of electricity.
II. Electrolysis Process
- During electrolysis, an external electrical source (battery or power supply) provides the energy to drive the non-spontaneous chemical reaction.
- Two electrodes (conductors) are immersed in the electrolyte solution:
- Anode: The positive electrode where negative ions (anions) are attracted and oxidized (lose electrons).
- Cathode: The negative electrode where positive ions (cations) are attracted and reduced (gain electrons).
- The specific products formed at the electrodes depend on the electrolyte used and the voltage applied.
III. Electrolysis of Water
- Water (H₂O) is a weak electrolyte, but it can be electrolyzed with the addition of a strong electrolyte like sulfuric acid (H₂SO₄) to increase conductivity.
- When an electric current passes through acidified water, the following reactions occur:
- At the anode (oxidation): 2H₂O (l) → O₂ (g) + 4H⁺ (aq) + 4e⁻
- At the cathode (reduction): 2H⁺ (aq) + 2e⁻ → H₂ (g)
- Overall reaction: 2H₂O (l) → H₂ (g) + O₂ (g)
- This process decomposes water into its constituent elements, hydrogen gas (H₂) and oxygen gas (O₂).
IV. Electroplating
- Electroplating is a technique for coating a metal object with a thin layer of another metal. It is a widely used industrial process for various applications.
- Electroplating involves the following steps:
- Electrolyte Selection: An electrolyte solution containing a dissolved salt of the metal to be deposited (e.g., copper sulfate for copper plating) is chosen.
- Electrodes: The object to be plated (cathode) and a bar of the plating metal (anode) are immersed in the electrolyte.
- Electrical Connection: The object to be plated is connected to the negative terminal (cathode) of the power supply, while the anode is connected to the positive terminal.
- Electrolysis: When the current flows, metal ions (cations) from the anode dissolve into the electrolyte. These cations are attracted to the cathode and gain electrons, plating them onto the object’s surface.
- Electroplating allows for precise control over the thickness and uniformity of the deposited metal layer.
V. Applications of Electrolysis
Electrolysis has numerous applications in various fields:
- Metal refining: Electrolysis is used to purify metals by separating them from impurities.
- Electroplating: As mentioned earlier, electroplating is used to coat objects with a thin layer of metal for various purposes like:
- Enhancing aesthetics (e.g., gold plating on jewelry)
- Corrosion protection (e.g., chromium plating on car parts)
- Improving electrical conductivity (e.g., copper plating on electronic circuits)
- Production of chemicals: Electrolysis plays a crucial role in the production of various chemicals, such as chlorine (used in water purification), hydrogen (used in fuel cells), and sodium (used in batteries).
- Electrolytic extraction of metals: Electrolysis is used to extract some metals from their ores. For example, aluminium is extracted from bauxite ore using this method.
VI. Important Points to Remember
- Electrolysis is a process where electricity drives a chemical reaction in an electrolyte.
- Electrolysis of water produces hydrogen and oxygen gas.
- Electroplating uses electrolysis to coat an object with a thin layer of metal.
- Electrolysis has diverse applications in metal refining, electroplating, chemical production, and metal extraction.
VII. Conclusion
The understanding of electrolysis has revolutionized various industries. It allows us to manipulate materials at an atomic level, leading to advancements in metal processing, chemical production, and technological development.
Electrolysis, Electroplating, and Applications
- What is electrolysis, and what type of substance is required for it to occur?
- Answer: Electrolysis is the process where an electric current drives a chemical reaction in an electrolyte (a substance that conducts electricity due to the presence of dissolved ions).
- What are the two electrodes called in electrolysis, and what happens to ions at each electrode?
- Answer: The electrodes are the anode (positive) and cathode (negative). During electrolysis, anions (negative ions) are attracted to the anode and undergo oxidation (lose electrons). Cations (positive ions) are attracted to the cathode and undergo reduction (gain electrons).
- Briefly describe the products formed during the electrolysis of water with sulfuric acid added.
- Answer: Electrolysis of acidified water produces hydrogen gas (H₂) at the cathode and oxygen gas (O₂) at the anode.
Electroplating
- What is electroplating, and what are some of its purposes?
- Answer: Electroplating is a technique for depositing a thin layer of metal onto another object using electrolysis. It’s used for various purposes like enhancing aesthetics, protecting against corrosion, and improving electrical conductivity.
- Explain the steps involved in the electroplating process.
- Answer: Electroplating involves choosing an electrolyte solution containing the desired metal ions, preparing the object for plating (cathode), using a bar of the plating metal as the anode, and connecting the electrodes appropriately to a power supply. As current flows, metal ions are deposited onto the cathode’s surface.
- How does electroplating allow for control over the thickness of the deposited metal layer?
- Answer: The thickness of the deposited metal layer in electroplating can be controlled by factors like the plating current, duration of the process, and the concentration of the metal ions in the electrolyte solution.
Applications of Electrolysis
- Besides electroplating, what is another application of electrolysis used in metal processing?
- Answer: Electrolysis is used in metal refining to purify metals by separating them from impurities.
- Give an example of a chemical produced using electrolysis.
- Answer: Chlorine, used in water purification, is a common chemical produced through electrolysis.
- How does electrolysis contribute to the production of hydrogen fuel?
- Answer: Electrolysis can be used to split water into hydrogen gas, a potential clean fuel source.
- Name a metal that can be extracted from its ore using electrolysis.
- Answer: Aluminium is an example of a metal extracted from its ore (bauxite) through electrolysis.
Understanding and Problem-Solving
- Why can’t electrolysis occur in pure water without adding an electrolyte like sulfuric acid?
- Answer: Pure water has very low conductivity due to minimal ions. Electrolytes like sulfuric acid increase the number of ions, allowing for the flow of electricity and facilitating electrolysis.
- In electroplating, if the anode dissolves too quickly, what might be the consequence?
- Answer: If the anode dissolves too quickly in electroplating, it can contaminate the electrolyte solution and affect the quality of the plated metal layer.
- Predict the observation at the anode if a copper sulfate solution is used for electroplating.
- Answer: During copper plating with a copper sulfate electrolyte, copper ions (Cu²⁺) from the anode will dissolve into the solution.
- Differentiate between the processes of electroplating and electrorefining.
- Answer: Electroplating deposits a metal layer on an object, while electrorefining purifies an existing metal by removing impurities through electrolysis.
- How does the concept of oxidation states relate to the reactions occurring at the electrodes during electrolysis?
- Answer: During electrolysis, oxidation (loss of electrons) increases the oxidation state at the anode, while reduction (gain of electrons) decreases the oxidation state at the cathode.
Applications and Real-World Connections
- Research and explain how electroplating is used in the jewelry industry.
- Answer: (Student research; answer may involve gold plating for aesthetics or rhodium plating to prevent tarnishing).
- Describe the role of electrolysis in the production of chlorine gas for swimming pool disinfection.
- Answer: (Student research; answer may involve electrolysis of brine solution to produce chlorine gas).
- Briefly discuss the environmental considerations associated with the production of certain metals using electrolysis.
- Answer: (Student research; answer may involve energy consumption or potential waste disposal challenges).
- How might advancements in electrolysis technology contribute to the development of more sustainable practices in the future?
- Answer: (Open-ended question; encourage discussion on potential applications like cleaner hydrogen fuel production or more efficient metal extraction processes).







Be the first one to comment on this story.